
Series of Basic biology for life science-2 What is the energy of life?
The seventh of the 17 Sustainable Development Goals (SDGs) set by the United Nations to create a sustainable world, is to overcome the energy crisis, and provide enough clean energy for everybody in the world. Inspired by this, the local government on Awaji Island plans to be entirely self-sufficient for energy needs, powered by renewable energy sources, including solar, wind, tidal, bio-mass (mainly from bamboo) and bio-ethanol. By 2018, island sources provided for 33 percent of the island’s energy needs.
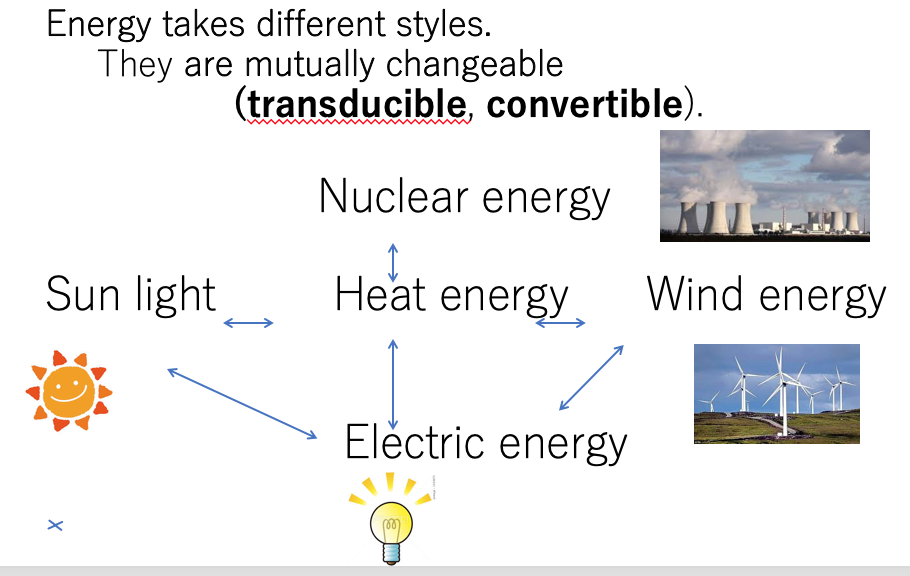
Energy is needed not only for cars, electronics, etc., but also for our bodies. In this article, we look at several basic points about energy, especially biological energy. We are familiar with various energy sources such as sunlight, nuclear, wind, etc. Sunlight through photo-conduction in special solar cells can produce electrical current. Nuclear power is released from energy trapped within the nucleus of an atom like Uranium. This power is used to boil water, and then make steam which drives turbines to produce electrical current. Energy exists in various places, from within single atoms to molecules where energy is used to form links between atoms. Wind energy is harnessed by wind turbines to produce electricity. As shown in Fig. 1, energy takes different forms, but can be converted among these forms. The universal unit of energy is the “joule”. 4.17 joules is the amount of energy required to increase 1g of water by 1 degree. Dictionaries define “energy” as the power required to do work.
To obtain energy for the biological work within our body, we eat foods, like rice and bread. Energy is stored within the starches of staple foods which in Japan is mainly rice. After starch is consumed it is digested by enzymes, amylase in saliva and then another amylase and maltase in pancreatic fluid, finally creating glucose. Glucose is taken up in the small intestine, enters the bloodstream and is then supplied to cells throughout our body. Glucose, containing 6 carbon, 12 hydrogen and 6 oxygen atoms, is the product of carbon dioxide and water combined with the solar energy of green plants. This solar power is converted and stored in the chemical bonds among the atoms within glucose. In the cells of our body, glucose is again broken down into carbon dioxide and water by oxygen. During this molecular separation of glucose, the energy stored in the glucose is released and transferred to an energy storage molecule ATP. The chemical structure of ATP, as shown in Fig.2, is different from that of glucose. The transferred energy is stored in between the second and third of three phosphorus atoms in ATP (Fig. 2) with a chemical bond energy of 4.7 Kcal(15.5 joule).
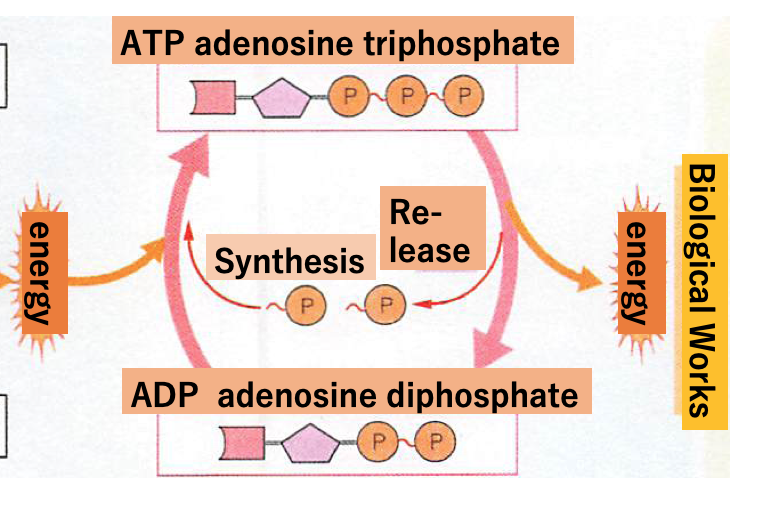
What is the energy in ATP used for? Biological work requiring energy can be classified into three categories. Category 1. Synthesis of component molecules into forms such as proteins; essential elements of our body called biological macromolecules (including proteins) are synthesized from elemental component molecules such as amino acids. Especially during infancy and stages of early human development, large amounts of energy in ATP is needed to make proteins by linking amino acids. However, adults also need ATP because our body’s proteins become degraded over time and need to be re-synthesized from amino acids (a process called metabolic turn-over). Metabolic turn-over is easily observed on the surface of the small intestine and skin. Category 2. ATP energy is needed for vision and motility. This energy is used for muscle contraction within eyes, legs and/or mouth, etc. Category 3. Maintenance of our body’s internal condition and cellular state at constant levels. One typical example is body temperature, maintained at around 36 to 37 degrees. Another example is the concentrations of ions like Na+, K+, Ca2+, etc. within cells and body fluids. The maintenance of such physical conditions is called homeostasis and needs ATP energy.
At rest, the rates of energy consumption of our body’s internal organs are 38 %, 12 %, 8 %, 6 %, 4 %, and 3 %, for muscle, liver, stomach and kidney, spleen, heart, and brain, respectively. However, once the body becomes active, this order of energy consumption changes. The brain now becomes the top, consuming 20 % of our body’s entire energy consumption. This suggests that without a good breakfast to supply energy to the brain, our activity level may be impaired.
Next, let’s take a look at how the energy in ATP is used for biological work. Muscle contraction is a good example. In muscles two proteins are involved in contraction, myosin and actin (Fig.3). Actin are globular proteins linked to each other in a chain-like structure. Myosin are formed as bundles with a part of each myosin molecule protruding in order to bind to an actin. The binding and release between the actin and myosin is reversible. The molecular structure of myosin changes depending on the presence or absence of ATP. Without ATP, a myosin protein will bind to an actin but once myosin binds to ATP, it eventually releases the actin after ATP is broken down. This phenomenon indicates that the energy stored in ATP is used to cause the change of molecular structure of myosin. By the way, the triggers that start and stop muscle contraction are signals from nerve cells. It should be noted that myosin is capable of binding to ATP and also performs an enzymatic activity similar to ATPase by decomposing ATP into ADP and Pi.
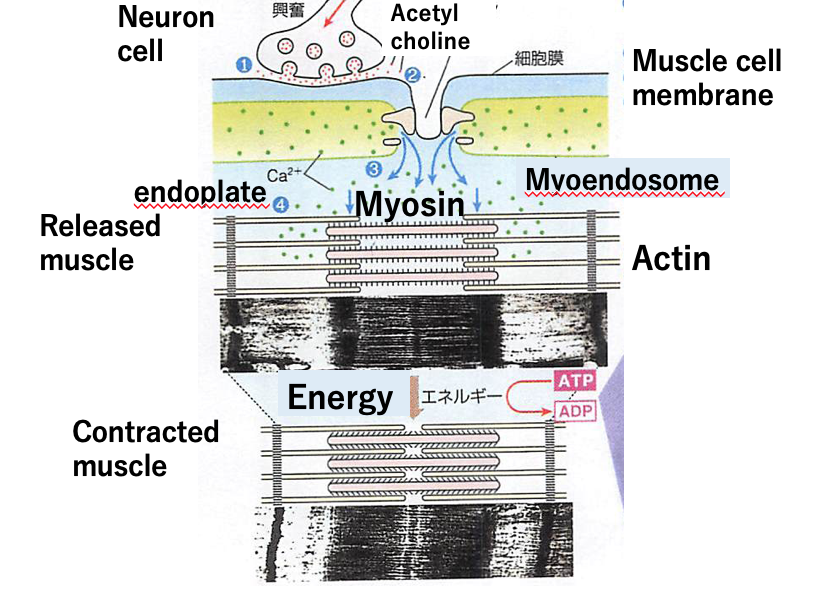
Most cases of biological work, including the functions of nerve cells, vision, and hearing, need a protein like myosin which is capable of binding and decomposing ATP. Similar to myosin, the energy in ATP is used for these cases as well. After ATP decomposes, the resulting ADP is restored to ATP by using energy from glucose in our meals.
