
Remember to watch the “C.L.O.C.K.”
The anti-aging effects of a regular diet.
A regular breakfast with the right balance of proteins and carbohydrates is known to support a healthy lifestyle. Now, a new report published in the prestigious journal, Science, supports this widely accepted wisdom with scientific data (A. Kumar et al. Brain-muscle communication prevents muscle aging by maintaining daily physiology. Science (2024) 382, p563). This paper describes the influence of “clock genes” on our health condition as we age. As Dr. Kumar describes, a regular diet supports the function of clock genes, leading to a host of health benefits, including a slowing of the ageing process.
An introduction to clock genes was provided on this HP previously (News 2023, April 18th). Let’s briefly review their role in our cells and body. Since we usually rest and sleep at night, ATP synthesis, the energy source in our cells, is mostly required during the daytime when we are most active. This mechanism requires many different proteins, mainly enzymes in mitochondria, which regulate the synthesis. Clock proteins provide the signals to start the process of ATP synthesis. In this way, the mechanism follows a logical pattern that matches the needs of usual lifestyles.
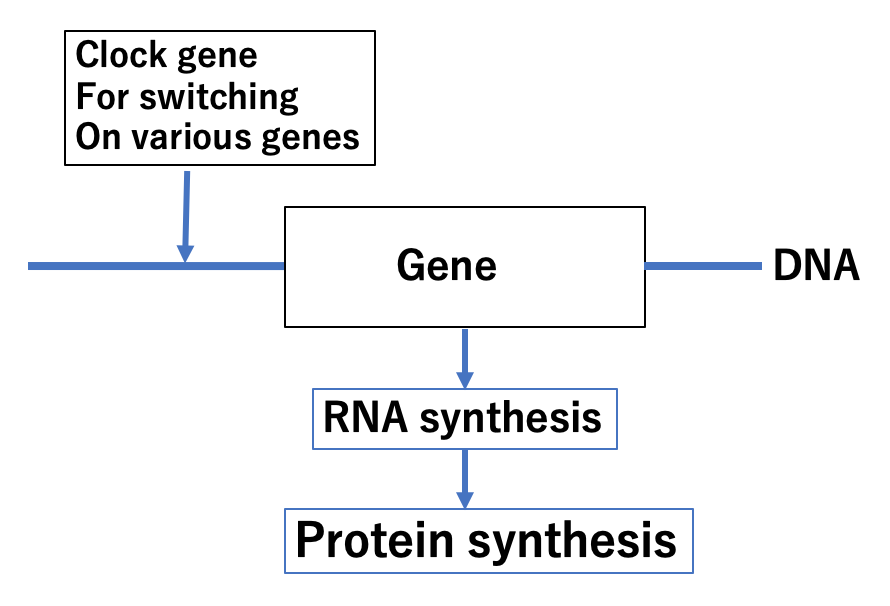
Fig. Clock proteins regulate protein synthesis as transcription factors.
Glucose and other nutrients, once taken in through meals, have to go through the cell membrane lining the surface of the digestive tract, transported by proteins integrated in the cell membranes. Proteins that facilitate this transfer, including “glucose transporters” also must be synthesized from late afternoon to early evening for energy production. Lipids taken up in the daytime are also used for energy production, but excess lipids are stored in the liver and consumed at night. The whole mechanism is under the control of clock proteins. However, if the daily cycle of clock proteins is hampered by mental and/or physical stresses, lipids may not be properly digested and in these cases are instead stored, leading to obesity.
Another example underlining the importance of circadian rhythms is sleep. The synthesis of melatonin, an essential hormone for sleep, starts from the pineal gland of the brain in the morning and accumulates through the day, reaching a peak just before bedtime. This process is also controlled by clock proteins. Aging, however, causes trouble in this cycle, leading to improper sleep times including delayed or premature sleep patterns. The cause of such problems is a functional decay of clock proteins, weakening their ability to adjust the timing of melatonin production.
What are the molecular mechanisms that govern clock protein functions? In all our cells, proteins repeat a cycle of increasing and decreasing synthesis every day. This cycle starts just after our birth and continues until our death. Some of these proteins, called transcription factors, are required for proper functioning of regular messenger RNA needed to synthesize proteins. The production of proteins like mitochondria enzymes, membrane transporters, and utilizing enzymes for lipids is regulated by these transcription factors which include clock proteins.
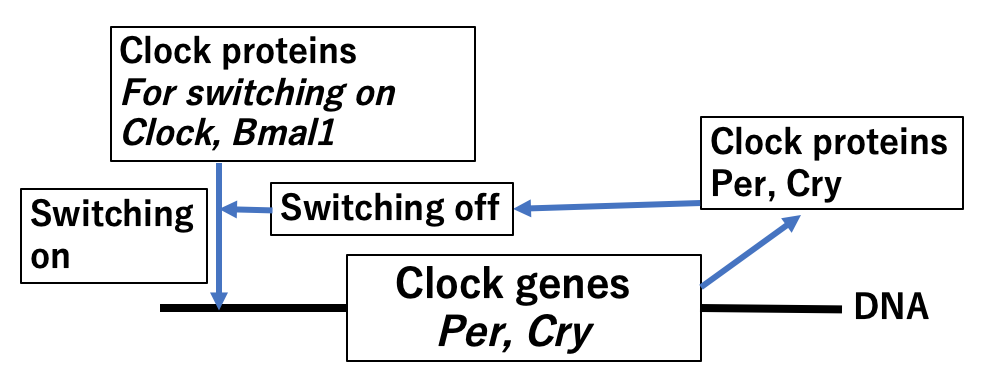
Fig. Molecular mechanism of circadian cycle by clock proteins
Clock proteins are classified into 2 groups. One group consists of “activator” transcription factors such as Clock and Bmal1 that switch “on” gene expression, and the other group includes “repressors” such as Per and Cry, for switching “off” gene transcription. Clock and Bmal1 proteins are synthesized all day and regulate the start of synthesis for mRNA of various enzymes (as described above) and also the clock proteins Per and Cry. Per and Cry are synthesized until night time, when they bind to Clock and Bmal1, subsequently supressing the activation functions of Clock and Bmal1 proteins on target genes. As a result, this mechanism in effect “switches off” the genes of various enzymes.
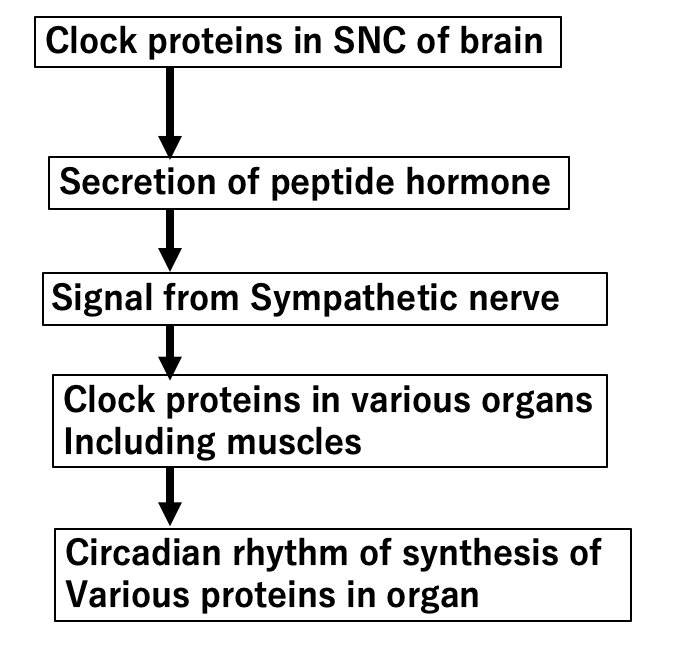
Clock proteins are distributed to all cells in our body. But a special set of clock proteins exist in a part of the central nervous system called the SNC (Suprachiasmatic Nucleus). Since SNC cells are located near the point where optic nerves from the right and left eyes intersect, SNC clock proteins are sensitive to light detected by eyesight. Under the control of clock proteins in the SNC, peptide hormones are produced and secreted from the SNC. One of these hormones binds to the pituitary gland and induces secretion of ACTH (adrenocorticotropic hormone), a stimulatory hormone that leads to the release of adrenalin from the adrenal grand. Adrenalin travels through the body, switching on the various mRNA of enzymes connected to many organs, including muscles. Another peptide hormone from SNC also binds to cells which control sympathetic neurons near the SNC. A signal from sympathetic neurons also induces the release of adrenalin, leading to a similar activation of parts of the body. Previously, it was thought that the circadian signals were received directly from the SNC to local clock genes in muscles and various other organs. However, the study by Kumar et al. shows that signals from the SNC can be selected by the local clock mechanism in the various organs.
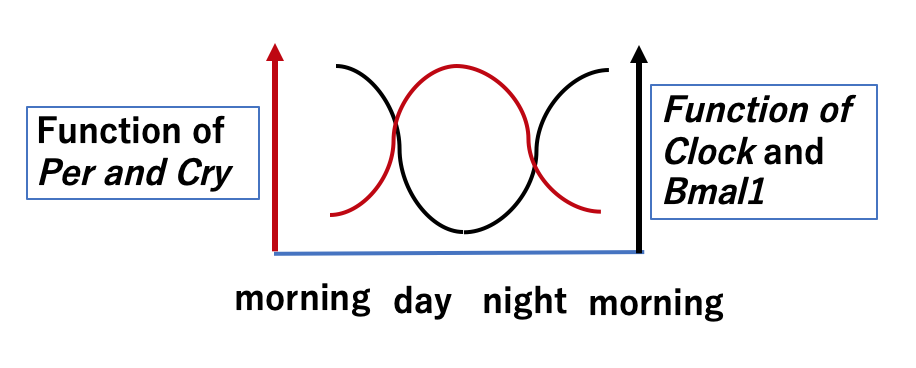
Circadian signals from the SNC seem to maintain stability, but can be sensitive to environmental changes. One common change is jet lag caused by the differences in the timing of sunlight. Changes in the circadian rhythm, including unusual times for meals may also lead to changes in blood glucose levels. Our internal environment is maintained at a constant level, called homeostasis, but is also affected by ageing, which brings a decrease in the activity of our clock. Kumar et al have shown that this decreased clock activity can be repaired through punctual meal times based on experimental trials using rats which had had their SNC clock genes removed. The rats were divided into 2 groups. One group were granted unlimited access to food, but the other group were fed under a strict feeding schedule. Indexed measurements of several activities of the subjects, especially muscle strength, indicated that those fed on a punctual schedule showed no loss of daily muscle activity, even without the clock genes of the SNC.
This illuminating research provides more concrete evidence of how lifestyle habits can affect health. The artificial lights and intense 24-hour rhythms of urban living often cause irregularities in the eating and sleeping routines of residents. A visit to places like Awaji Island nestled in the cradle of nature may be one of the best ways to reset our “clock” for a longer, healthier life.
