
Why do we need salt for our life?
Awaji Island used to be one of the major areas for salt production about 1,500 years ago or more.
In an ancient time, people made salt from sea water by washing sea weed and then condensed salt by heating the washed water. Earthen vessels for heating sea water have been found in several remains in Awaji Island, like at Kifune Shrine area of the northern Awaji Island and Aman in South Awaji City. However, unfortunately these processes and skills for salt production were lost a long time ago. Later other methods for salt production were invented by applying condensation of sea water with other techniques. These processes were again lost in the late Edo period before the Meiji period started.
Now, major salt production from sea water uses filtration of sea water by a special membrane to remove most of the materials in sea water except NaCl (sodium chloride). Based on this new technique almost pure sodium chloride (NaCl) is industrially produced. This type of salt contains more than 97 % pure NaCl. Salt thus means NaCl itself now. However, we need not only NaCl but also other salts (inorganic ions) are needed for our life.
Here topics in terms of salts essential for our life, especially NaCl is picked up. “You should not send salts to your enemy”, a famous phrase in the middle age of Japan, is symbolic to show an essentiality of salt for our life. In our body fluid, especially blood, 140 mg / litter NaCl is contained which is consistent with the salty taste of blood. On the other hand, in the fluid inside of cells including red blood cells NaCl concentration (15 mg/litter) is about one tenth of that in the outside cells. NaCl concentrations outside and inside cells are maintained at a certain level for each. One example when the maintenance of the concentrations is disturbed is a heat stroke to our body like during the hot summer season. In this situation, body salts are lost by higher level of perspiration leading to lowered brain functions and then sometimes more serious symptoms. When we take more salts, blood salt level increases and then more water comes into blood, leading to high blood pressure which might cause burst of blood vessels. The Japanese Ministry of Health and Welfare recommends 6 grams of NaCl / day. WHO indicates that suitable NaCl intake is less than 5 grams /day. But most Japanese take more salt, about 10 grams / day. A reason of this high salt intake is based on Japanese dietary habits, which are dependent on rice as a staple food.
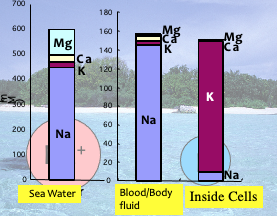
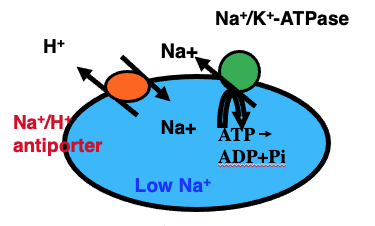
NaCl concentration outside cells are higher than inside as described above. Interestingly, the outside salt composition and concentration ratios of various ions, especially Na+, Mg2+ and K+, are similar to those in sea water (Fig. 1). This may reflect an instinctual memory of environment in our body which the origin started from sea water. Here, we see biological importance of the concentration-difference of NaCl between the outside and inside cells. Cells are surrounded by biological membranes in which major chemical components are lipids. Nutrients like sugars, amino acids and ions are mostly water soluble and cannot penetrate the biological membranes. But many proteins are integrated in the membranes and provide for pathways of nutrients and ions, named membrane transporter proteins. One of the transporters called a pump, can pump Na+ ions from the inside to outside cells as shown in Fig. 2 (shown as Na+/K+ ATPase) using biological energy substance, ATP. Some of the membrane transporter proteins can transport Na+ ion from the outside to inside cells depend on the concentration gradient of Na+ ions. Those transporters can transport nutrients from the outside to inside by using the flow energy of Na+ through the same transporter protein (Each of many transporter proteins integrated in the membranes can transport a substance specific to each transporter proteins shown in Fig. 3). One of the transporter proteins can transport glucose from our meals to the inside of cells by such mechanisms. Na+ ions taken into inside cells are transported to the outside again by Na+/K+ ATPase as shown in Fig. Na+ ions are recycled to make a flow of the ions. This Na+ ion flow is also essential for the nerve cell function. These are reasons why we need salt (NaCl), as an essential substance for our lives. Nakamura and Kanazawa found a new type of such transporter which transport H+ ion by using the flow of Na+ ions (N. Nakamura et al (2005) J. Biol. Chem., 280, p1561). Eventually malfunction of this transporter protein was found to be causative of mental retardation for boys, because the gene for this protein exists on X chromosome. (Y. Takahashi and S. Saito et al. (2011) Am. J. Med. Genetic. , Part B, p799).

Sodium chloride (NaCl) is a chemical molecule made of Na+ and Cl–. Therefore Cl– also plays an important function. One Cl– transport protein exists in membranes of epithelial cells of the lung tube. Malfunction of this protein causes unusual Cl– ion balance between the outside and inside of cells, leading to accumulation of excess water in the lung tube. This causes cystic-fibrosis, one of the most frequent inherited syndrome for children of Caucasian people who die because of serious trouble in respiration.
As described above, besides Na+ ions Mg2+, Ca2+, K+ ions together with several other ions are essential for us. Those play also important roles in our body like NaCl. Therefore, NaCl alone is not good enough for us. Sea water contain MgCl2 (10%), MgSO4 (6%), KCl(4%), CaSO4 (2%) beside NaCl (78%). Because of this feature, one person who moved from outside of Awaji-Island started to produce salt from sea water by using classic method as described above. The salt is commercially available as “onokoro-sizuku salt”. This is a nice example of new challenges in Awaji Island which may activate local activities and contribute to the traditional culture of the island.
